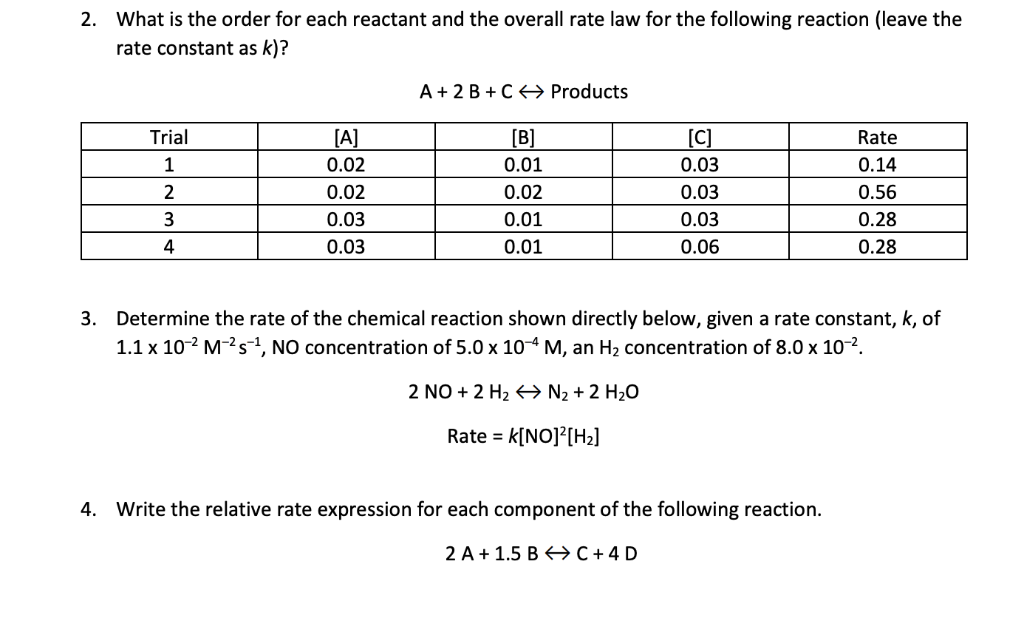
![Which of the following rate laws has an overall order of 0.5 for the reaction involving substances x, y and z? ([Cx],[Cy] and [Cz] respectively are the concentrations of x,y and z) Which of the following rate laws has an overall order of 0.5 for the reaction involving substances x, y and z? ([Cx],[Cy] and [Cz] respectively are the concentrations of x,y and z)](https://haygot.s3.amazonaws.com/questions/1958686_661431_ans_54de31a89c134e31baf64d6b1781bccb.jpg)
Which of the following rate laws has an overall order of 0.5 for the reaction involving substances x, y and z? ([Cx],[Cy] and [Cz] respectively are the concentrations of x,y and z)
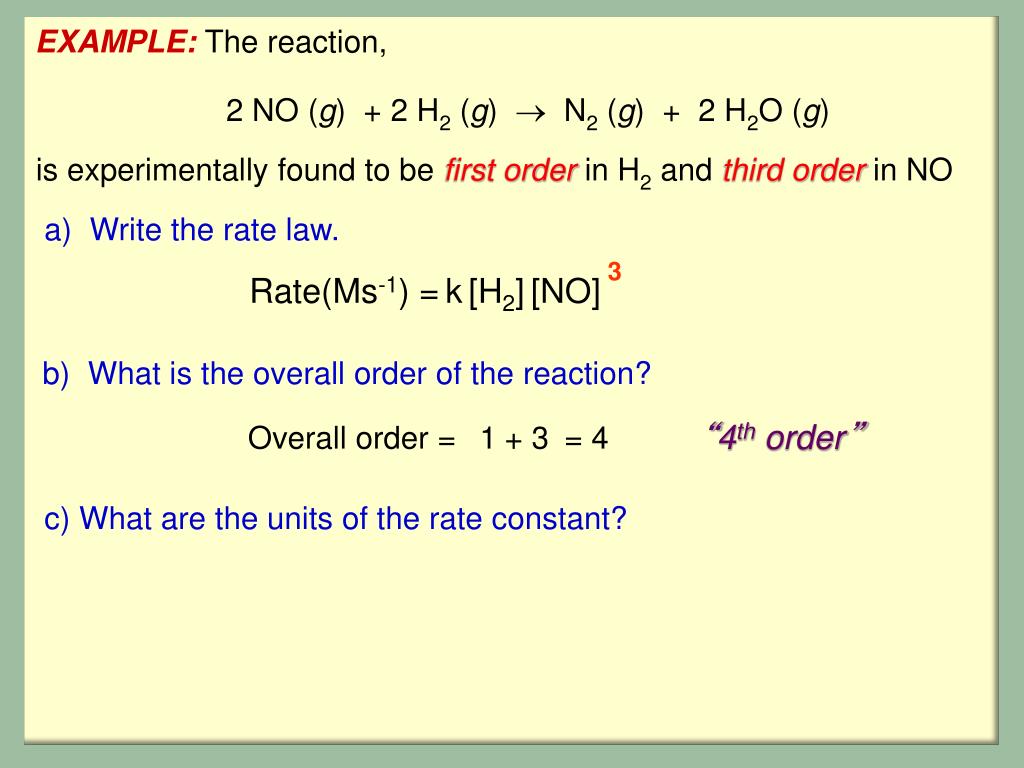
PPT - Chapter 15 Chemical Kinetics: The Rates of Chemical Reactions PowerPoint Presentation - ID:2436869

The following data are for the reaction A + B→ products :Conc. A Conc. B Initial Rate(M) (M) ( mol L^-1 s^-1 )0.1 0.1 4.0 × 10^-4 0.2 0.2 1.6 × 10^-3

A rate constant for a particular reaction is 0.0050 s-1. What is the overall order of this reaction? | Homework.Study.com

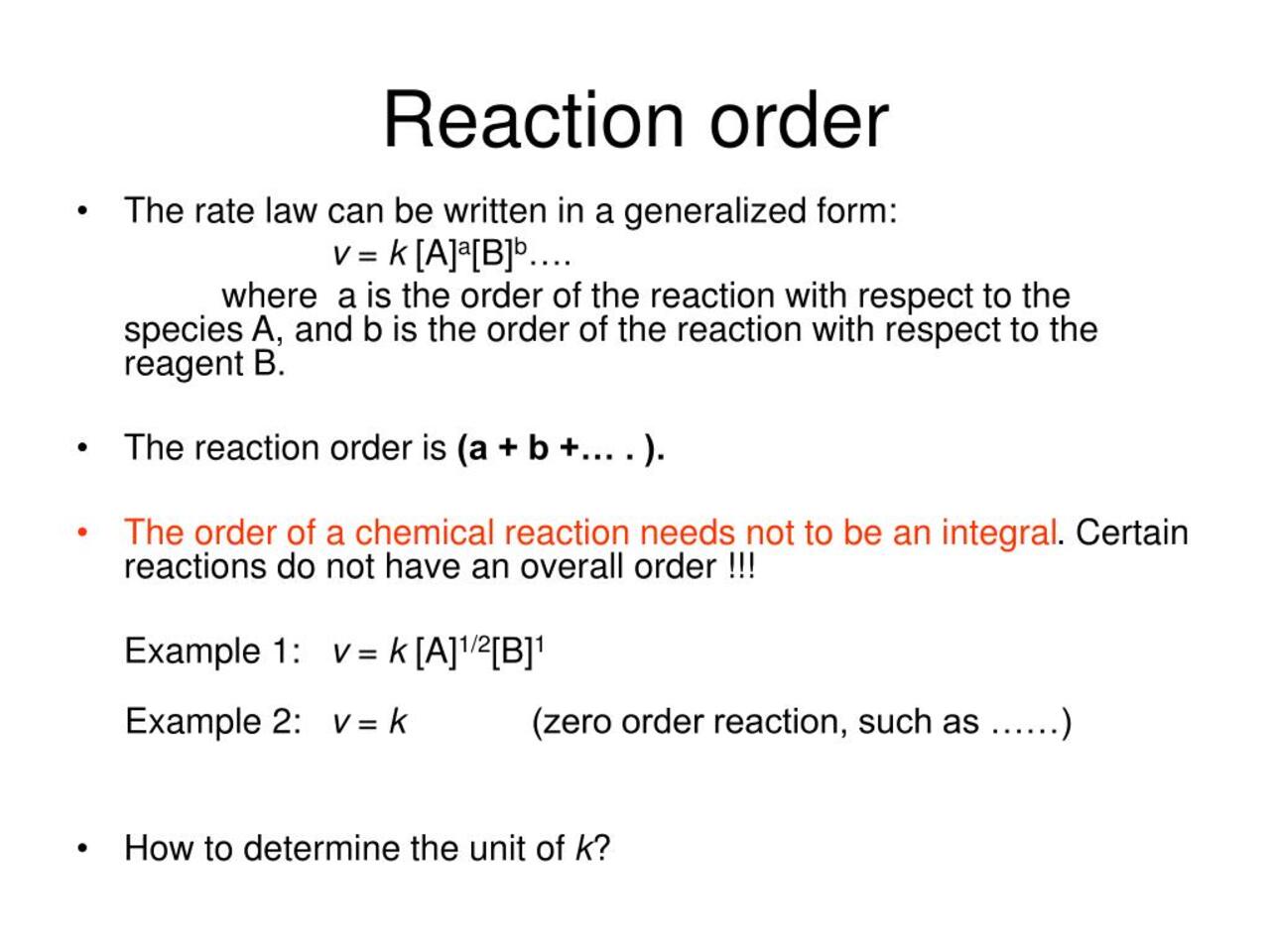
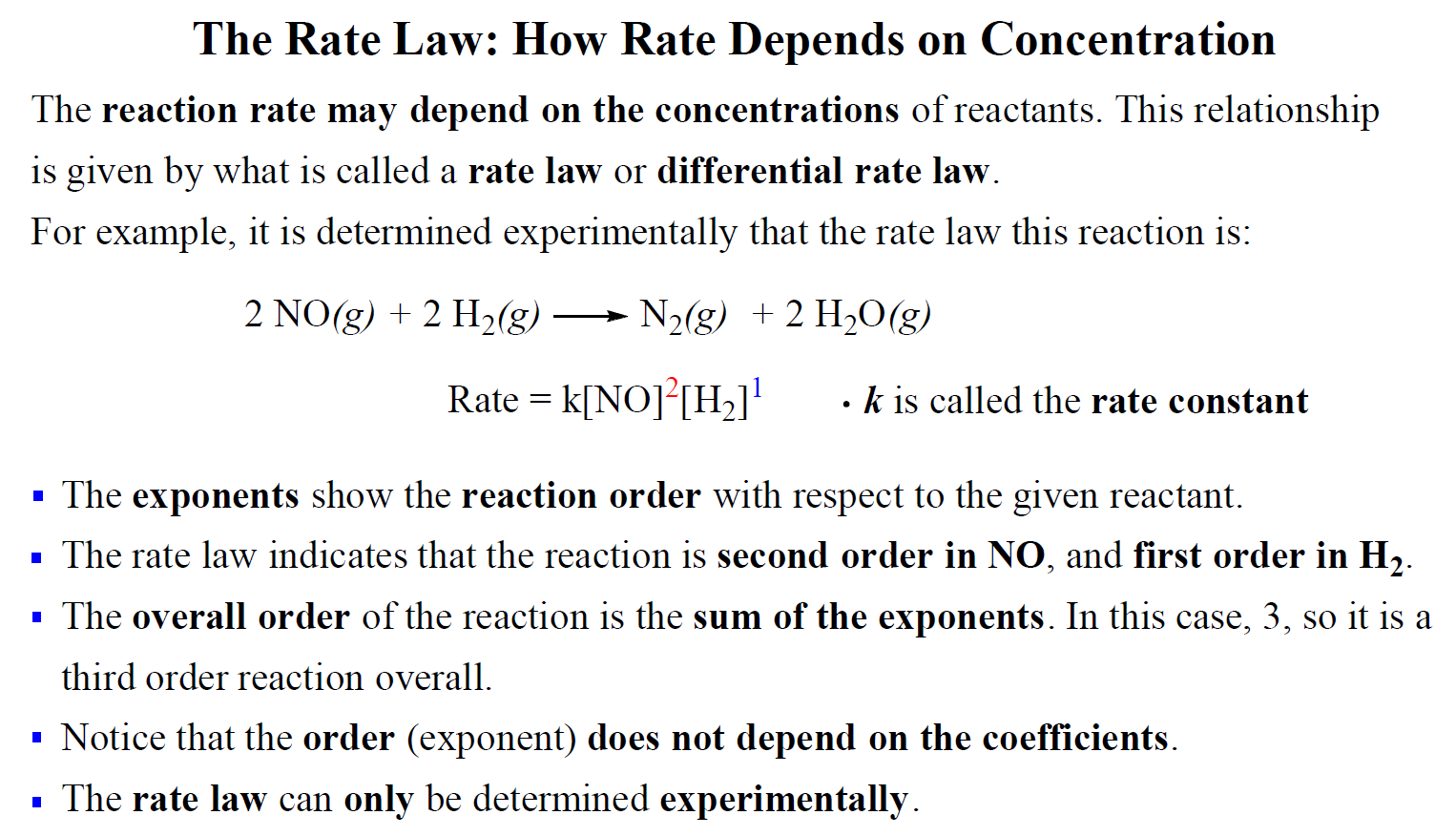


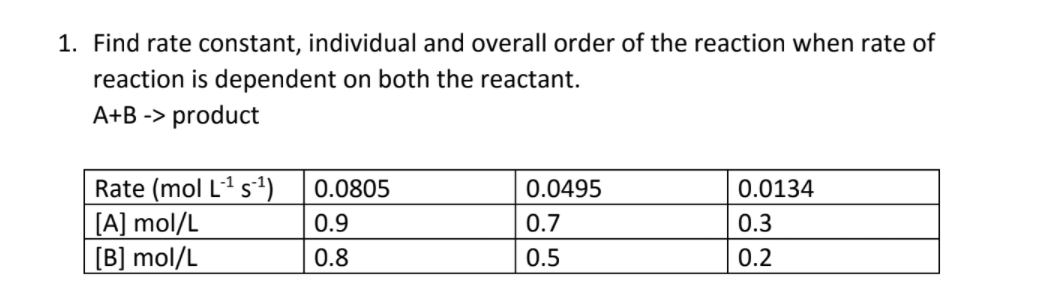



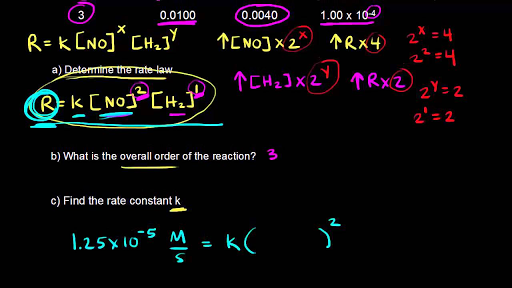
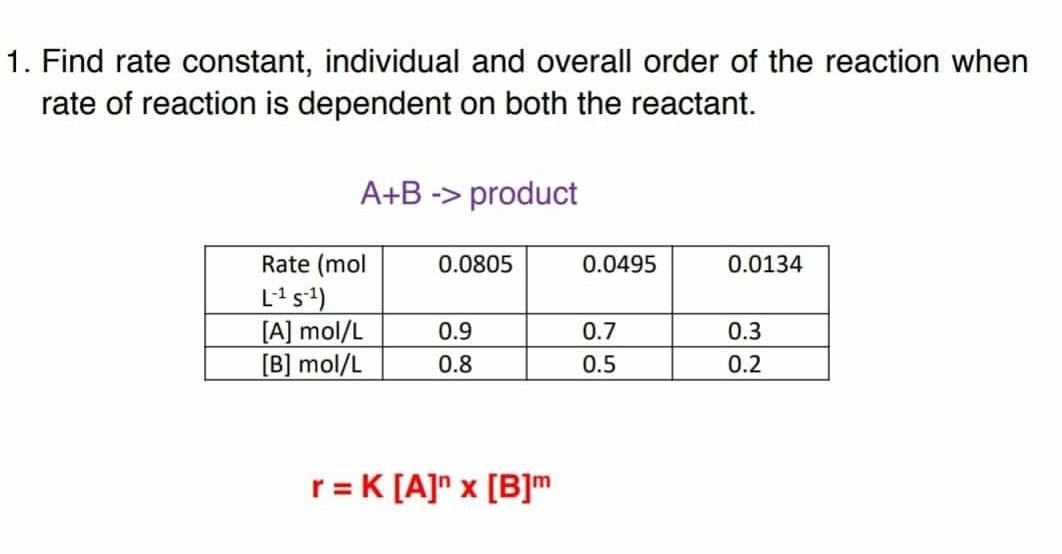

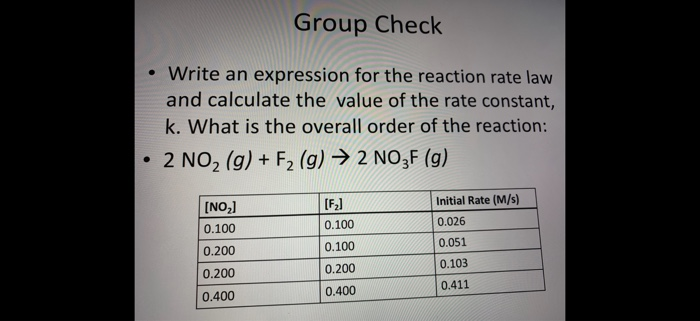
![16.1/R2.2.6 Rate constant, overall order of reaction, order of reaction [HL IB Chemistry] - YouTube 16.1/R2.2.6 Rate constant, overall order of reaction, order of reaction [HL IB Chemistry] - YouTube](https://i.ytimg.com/vi/9sMFJMuZzmg/sddefault.jpg)